Determination of the vapor pressure of liquid and solid test substances using the static measurement method (OECD 104)
According to EU Regulation (EC) No 1907/2006 – Registration, Evaluation, Authorization and Restriction of Chemicals (REACH), chemical substances may only be marketed in the EU if they have been registered beforehand. For all substances that exceed an annual production quantity of one metric ton, a technical dossier must be submitted in this context which contains the vapor pressure of the substance in addition to other safety data.
The vapor pressure of a liquid or a solid is the pressure that results when the gaseous phase and the respective condensed phase are in equilibrium. Equilibrium between the liquid and gas phase (vapor pressure curve) depends on the temperature. The vapor pressure increases as the temperature rises.
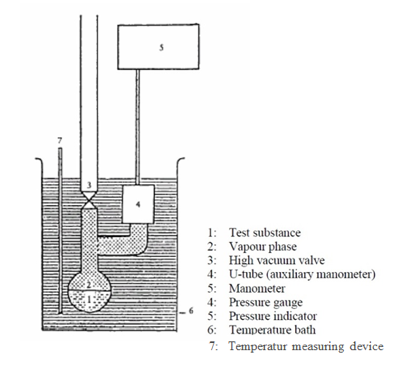
Test setup in accordance with the OECD 104
The key condition for use of the static measuring method to determine the vapor pressure is that equilibrium must be established at a defined temperature before the respective measurement. This means that pressure, volume, temperature and concentration of the substance must be constant at the time of measurement. The Organization for Economic Cooperation and Development (OECD) also requires a maximum deviation of 10% as well as a temperature stability of 0.2K be guaranteed for the validation of the measurement method. The consilab laboratory has an apparatus that been developed to fully meet the requirements. With this apparatus, vapor pressures ranging from 133 Pa to 2.6105 Pa can be measured over a temperature range of 40-200°C.
To determine the vapor pressure, the sample is filled into the test apparatus, where it is degassed in situ through cyclic pumping. The entire apparatus, including the pressure sensor, is then heated to the desired temperature. After the target temperature has been reached and the vapor-liquid equilibrium has been established, the vapor pressure is measured at the controlled temperature. A new target temperature can then be activated. Following this procedure, the vapor pressure curve for the test substance is determined in increments.

The vapor pressure curve of cumene as measured at consilab compared with the relevant literature data
In addition to the measuring method described here, the consilab laboratory also has two other apparatuses for determining vapor pressure that can be used to perform measurements in other temperature and pressure ranges.
